Rx only • For subcutaneous use only • United States
Ganirelix Acetate Injection 250 mcg/0.5 mL
Product information for patients, healthcare professionals, and wholesalers/pharmacies — including Prescribing Information (PI) and contact for inquiries.
Indication
Ganirelix Acetate Injection is indicated for the inhibition of premature LH surges in women undergoing controlled ovarian hyperstimulation.
For Patients
This page provides product information and resources. Please speak with your healthcare provider about whether this product is appropriate for you. For complete information, see the Prescribing Information.
For Healthcare Professionals
Quick access to indication, key labeled administration highlights, how supplied, and Prescribing Information.
For Wholesalers / Pharmacies
NDC, presentation, storage details, and an inquiry form for ordering or distribution questions.
Important Safety Information (ISI)
The following is a brief summary. Please see the full Prescribing Information for complete details.
Contraindications
- Known hypersensitivity to ganirelix acetate or any of its components.
- Known hypersensitivity to GnRH or any other GnRH analog.
- Known or suspected pregnancy.
Warnings & Precautions
- Should be prescribed by physicians experienced in infertility treatment.
- Before starting therapy, pregnancy must be excluded.
- Hypersensitivity reactions (including anaphylaxis, angioedema, and urticaria) have been reported; discontinue and treat if suspected.
Common Adverse Reactions (≥1%)
Abdominal pain (gynecological), fetal death, headache, ovarian hyperstimulation syndrome (OHSS), vaginal bleeding, injection site reaction, nausea, abdominal pain (gastrointestinal).
Report Adverse Events
To report SUSPECTED ADVERSE EVENTS, contact Mullan Pharmaceutical Inc. at +1-800-673-9839 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Prescribing Information
Download Prescribing Information (PDF)
Please see the full Prescribing Information for complete details.
Patients
What is Ganirelix Acetate Injection used for?
Ganirelix Acetate Injection is indicated for the inhibition of premature LH surges in women undergoing controlled ovarian hyperstimulation. For complete information on use, risks, and instructions, please see the Prescribing Information and talk with your healthcare provider.
Rx only. For subcutaneous use only.
Directions for Use (Injection)
The following steps summarize the labeled instructions. Follow your healthcare provider’s directions and the full Prescribing Information.
Healthcare Professionals
Indication (US)
Ganirelix Acetate Injection is indicated for the inhibition of premature LH surges in women undergoing controlled ovarian hyperstimulation.
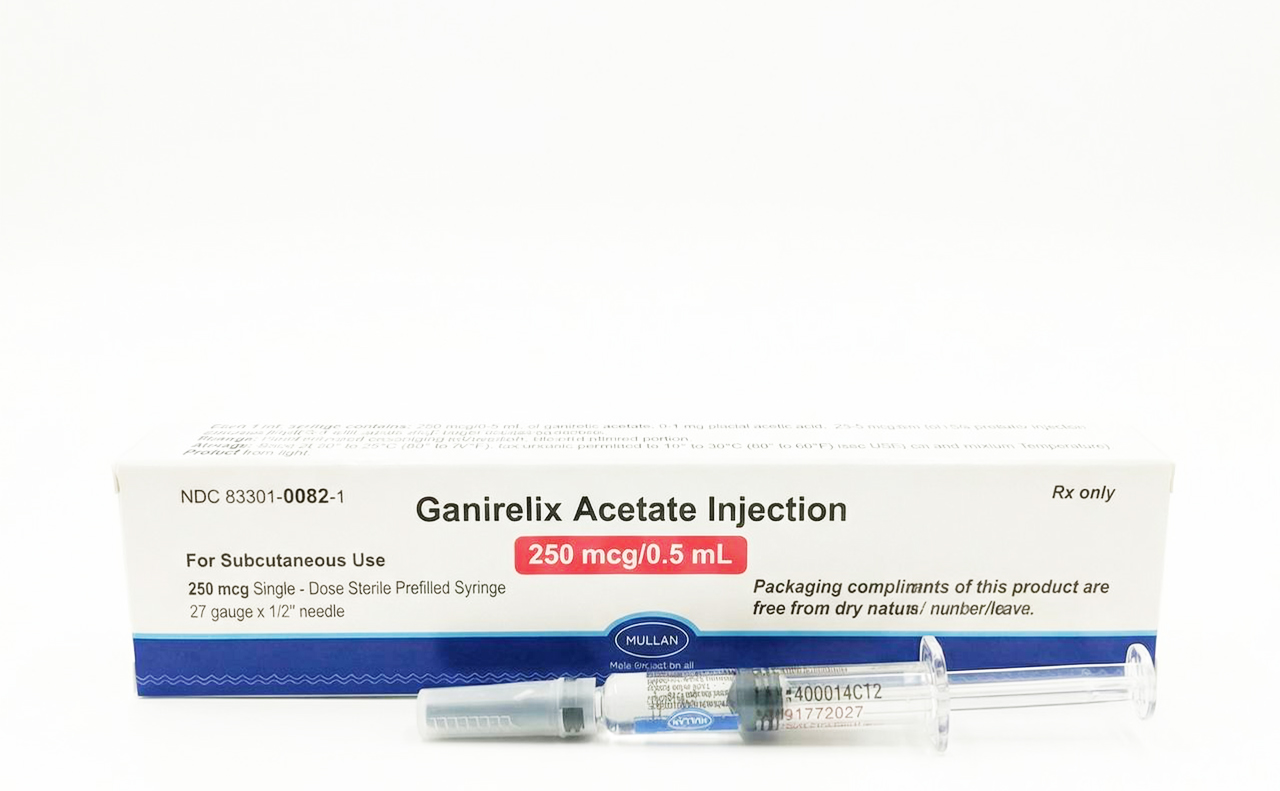
How supplied
- Single-dose, sterile, prefilled 1 mL glass syringe containing 250 mcg/0.5 mL aqueous solution.
- Affixed needle: 27 gauge x 1/2-inch.
- Latex statement: Packaging components are free from dry natural rubber/latex.
Dosing & administration (see PI for full details)
After initiating FSH therapy on Day 2 or 3 of the cycle, Ganirelix Acetate Injection 250 mcg may be administered subcutaneously once daily during the mid to late portion of the follicular phase and continued daily until the day of hCG administration.
Storage
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F). Protect from light.
Wholesalers / Pharmacies
Product details
- NDC: 83301-0082-1 (single syringe)
- Presentation: 250 mcg/0.5 mL single-dose sterile prefilled syringe
- Storage: 20°–25°C; excursions 15°–30°C; protect from light
Questions / distribution inquiries
Use the inquiry form below and select “Wholesaler/Pharmacy” for faster routing.
Contact / Inquiries
Submit the form below and our team will respond.
